BIOLOGICS AND BIOSIMILARS WEBCAST
Your questions answered
This is the transcript of our 2017 webcast on biologics and biosimilars.
Biologics and biosimilars are a hot topic at the moment.
In 2017 we held a webcast to answer your questions on the topic. We had a huge response with many questions streaming in.
For those of you who haven’t heard of biologics, they’re a group of medications that can help reduce your immune response towards the tissues of your own body, helping to reduce irreparable joint damage from conditions such as rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis. What makes them different to older medicines is that they’re cultured in living cells or tissues rather than being synthesised in the laboratory. Unlike traditional drugs, which often have generic versions or copies, we cannot be sure that the copy of a biologic, called a biosimilar, will be 100% identical to the original brand version.
To keep everyone up to date here is the transcript of the broadcast which was delivered by Ms Naomi Creek who coordinates our Young Women with Arthritis Peer Support Group leader and long-time MSK Ambassador, Prof. Rachelle Buchbinder, rheumatologist and Director of the Monash University Department of Clinical Epidemiology and current president of the Australian Rheumatology Association and A/Prof Michael Ward, Discipline in Pharmacy at the University of South Australia and member of the Advisory Committee on Medicines Scheduling of the Therapeutic Goods Administration.
Definitions
- Antibody – part of our immune system and they help fight off antigens.
- Antigen – a substance our body recognises as foreign, and starts an immune response.
- Anti-drug antibody – an antibody against a specific drug.
- Enzyme – proteins that are produced by cells to do a specific action. Each type of enzyme is created to do one specific thing, for example breaking down starch to sugar, cutting up DNA, digesting drugs, or binding molecules together. Without enzymes our bodies wouldn’t be able to function.
- IgG antibody – there are five types of antibodies, IgG, IgM, IgA, IgE, and IgD. They’re distinguished according to what molecules they’re made up of. These molecules determine how they work. The IgG antibody (otherwise referred to as Immunoglobulin G) is the most common antibody in the human body, it crosses the placenta from mother to fetus and protects against bacteria and viral infections.
- Immunogenicity – the ability of a substance foreign to the body (an antigen) to provoke an immune response by the body against it.
- Pharmacodynamics – the branch of pharmacology that studies the action and effects of medicines.
- Pharmacokinetics – the study of how drugs act in the body, how they’re utilised, how fast, how long they last and rate of excretion out of the body.
Your questions
- Biologics and biosimilar introduction
- At what point should I start taking a biologics medication and what damage could be caused if I don’t take these medications because of the pain and inflammation that is not being treated?
- How long would someone have to be on a biologic for before they notice a benefit?
- Do you have to be on biologics long term? Potentially what are the side effects of someone being on a biologic in the short and long term?
- If someone is in remission and they then decide to stop taking the biologic or biosimilar what may happen then?
- I know a lot of people go from one biologic to another biologic because they stop working. Why do they stop working?
- If they did stop working, would they also perhaps have their dose increased to see if that becomes more effective for them?
- What is the record for some of these biologics working really well for?
- Is there much evidence of effective use of these medications specifically in ankylosing spondylitis?
- Do biologics affect hormone levels in any way? In particular testosterone and cortisol?
- Do biologics affect the liver and kidney?
- Compared to other medications are biologics more risky?
- What about people who are considering having a family? What do they need to know about biologics? Can they be on these before getting pregnant, and after and during breastfeeding? And also males, what do they need to consider as well?
- What other medications were there that people have been on during pregnancy? You mentioned Enbrel®, what are some of the others?
- Can biologics such as Humira® have an adverse effect on other medications such as anti-depressant medication?
- Are children given biologics or biosimilars?
- What is the success rate of Humira® for rheumatoid arthritis as opposed to using Enbrel® and Xeljanc®?
- Do biologics have any effect on insulin action and blood glucose levels?
- What is the accessibility like for, or the eligibility for biologics and what does a patient need to do to be able to get onto a biologic or a biosimilar?
- How biosimilars are evaluated
- What are the risks then for someone switching if they have been stabilised on a medication, on a biosimilar and then are switched?
- What are substitution and switching?
- What should patients do to make sure they keep abreast of their condition and their medication in relation to switching at the pharmacy level?
- Would patients learn about injecting devices that may differ between the biologic and the biosimilar from their rheumatologist or would they talk to their pharmacist?
- Some biologics are a given in hospital settings through intravenous therapy (IV). What should patients be aware of or ask questions about – with just keeping on top of what’s being put into them?
- Is there a risk of patients not knowing a switch occurs in a hospital setting?
- If more patients take biosimilars in the future does this mean that rheumatoid arthritis sufferers will be able to get on these drugs more quickly without waiting to try other drugs first?
- If a patient has been on several biologics over 6+ years but blood inflammation levels (ESR and CRP) remain high (e.g.: 35-40 for over a year) what is the probability of the government withdrawing the subsidy if these levels are still high and the drugs are not working for them?
Answers
Biologics and biosimilar introduction
Rachelle: To understand biosimilars we firstly need to understand biologics. Biologics are medicines whose active substance is a very large, complex structure that can only be made from living organisms. So bacteria or a yeast or a human or animal cell line. So it is a living substance. This includes biologics such as Enbrel®, infliximab and many of the other biologics that we use to treat our inflammatory arthritis conditions. There are also other biologics, including low molecular weight heparin, some hormones and enzymes for people with enzyme deficiency.
What a biosimilar is, is a product similar to the biologic in terms of how it works and its effectiveness. However while it is similar to a biologic it is not an identical copy. It has the same backbone but made using different cell lines so it won’t be 100% identical.
If we wanted to make something like aspirin it would be relatively easy for someone in the lab to make it because it is synthetic and it has a very simple structure and it would be exactly the same as the original. That’s what we call a generic medicine. Generics are usually cheaper than the original but they are identical in all ways because they are made synthetically.
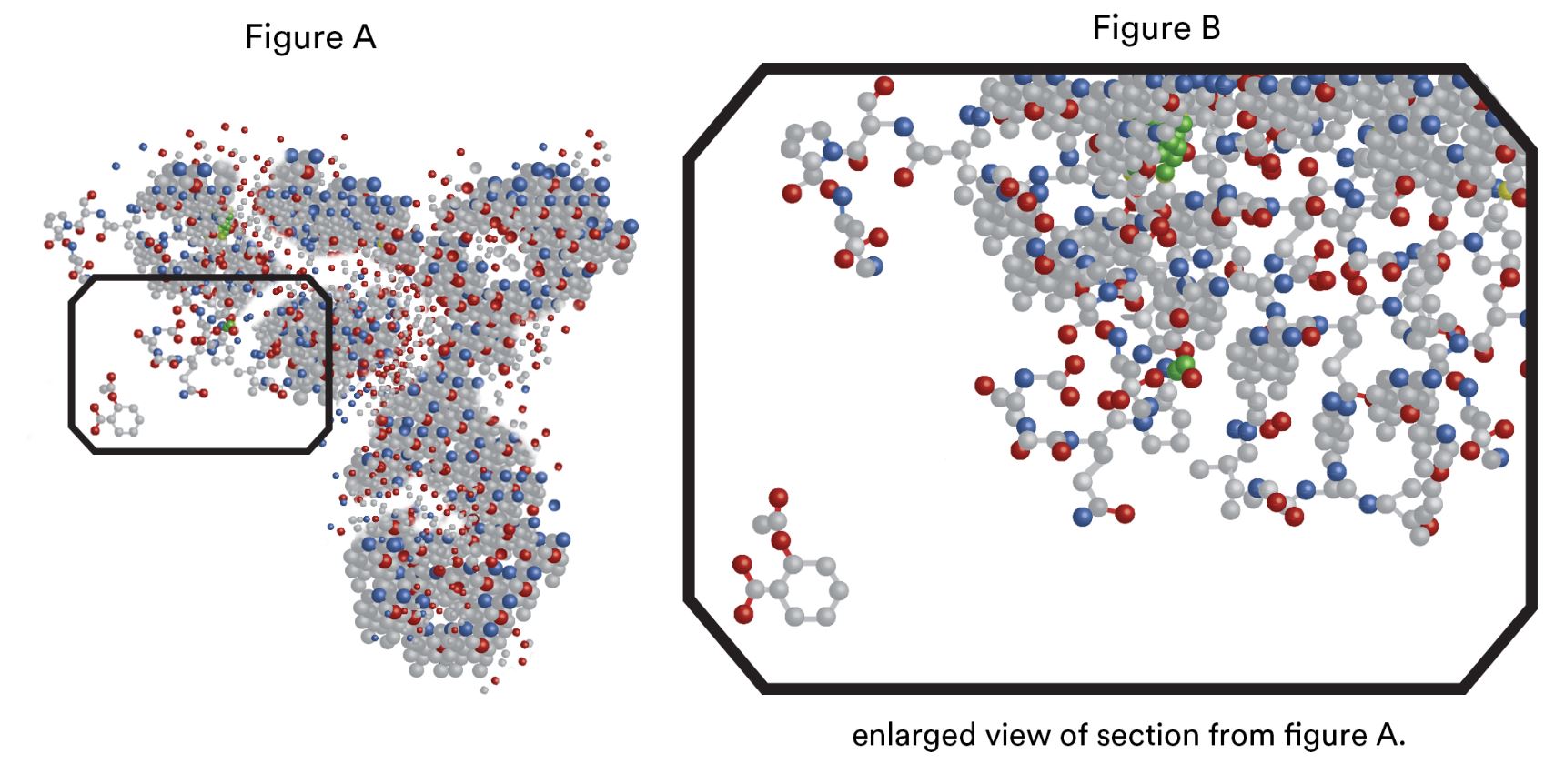
If we have the next slide [Diagram 1] I can show you why it is so hard to make biologics identical. And not even a new batch of the same biologic will be identical to the previous batch. So, the complex structure that you see is an IgG antibody. As you can see it looks very complex. In the next slide we will focus on the rectangle.
Each of these grey structures are the amino acids that make up the antibody. And the little simple structure on the left bottom that’s aspirin.
So I hope you can appreciate how easy it would be to copy something like aspirin, but how hard it would be to copy something like a biologic. And this representation does not do justice to the three dimensional structure with different bits folded in on itself. So it’s a really, really complex structure that you can’t make exact replicas of.
In Australia we have very strict rules about which drugs are able to be offered to patients through the TGA, and which can be subsidised through the PBS. In general to be subsidised on the PBS, a drug must be shown to be at least as effective and/or safe as another drug that is already available, and at the same or lower cost. This is the process that was followed to get PBS-subsidised biologics. The pathway is much simpler for a biosimilar but it varies country by country. Usually they also have to demonstrate efficacy, and have data about their pharmacodynamics, pharmacokinetics, and immunogenicity, which refers to their ability to elicit an immune response.
They have to be comparable to the originator but they don’t have to go through the very strict clinical trials that the biologics have had to. Part of the reason for that is that people generally think they are very similar although not identical and they are also cheaper.
Naomi: At what point should I start taking a biologics medication and what damage could be caused if I don’t take these medications because of the pain and inflammation that is not being treated.
Rachelle: So we now have very great drugs to treat RA. We have the traditional oral drugs such as methotrexate (which can also be given by injection), the newer biologics, and now some biosimilars. The point at which you should start biologics really depends on whether or not you are well controlled on the older drugs. In Australia we have a very strict process for getting biologics and you have to have failed to respond to at least 3 of the older drugs first. Having said that, many people can be well controlled on the old drugs and may never need a biologic. The bottom line is you need the medication when the medication you are currently on fails to adequately control your symptoms and disease. The most important thing is to prevent damage to the joints. We want to put your disease into remission as much as we are able, and that will also reduce the risk of long term complications from the disease. The modern approach is something we call ‘treat to target’; we want to suppress the disease to below a certain level and if that is not happening then we need to escalate the treatment.
Naomi: How long would someone have to be on a biologic for before they notice a benefit?
Rachelle: Different biologics take different amounts of time to work. Some of them work very, very, quickly, sometimes in a day, some take a month, and some take several months
Naomi: And would someone need to be on them long term? And potentially what are the side effects of someone being on a biologic in the short and long term?
Rachelle: This very much varies by which biologic or biosimilar you are taking. For self-injectable biologics or biosimilars, the most common side-effects are local skin reactions, but these usually get better over time. Most people don’t need to stop the drug because of this. There are range of other more serious side effects but if picked up early, the drugs can be stopped and a different drug can be tried. At the moment we don’t have any drugs that cure RA. Although there are exciting products in development such as vaccines that might do that in the future. So at best we suppress the disease. Having said that, some people do go into remission spontaneously and some people are able to increase the time interval between treatments and sometimes stop them altogether when they are on biologics as well.
Naomi: If they are in remission, and they then decide to stop taking the biologic or biosimilar what may happen then?
Rachelle: The drugs suppress the disease but they don’t cure the disease so stopping the drugs risks the disease coming back. Some people might notice a flare up very quickly if they stop their drug or even forget to take it on time. In other instances it might take longer for the disease to flare.
Naomi: I know a lot of people go from one biologic to another biologic because they stop working. Why do they stop working?
Rachelle: We run a national biologics database, called Australian Rheumatoid Association Database (ARAD) and hopefully most of the listeners are in that database. We know a lot about why people might stop taking a drug. We know that about a half of all people that start taking one drug will remain on it and the other half will stop it for a variety of reasons, such as side-effects, or it stops working. No one really knows why these drugs can sometimes suddenly stop working after a long period of time although in some it may be due to the development of antibodies which interfered with their action.
Naomi: If they did stop working, would they also perhaps have their dose increased to see if that becomes more effective for them?
Rachelle: In Australia we don’t usually have the capacity to increase the dose. If they are on a reduced frequency of treatment they could go back to the prescribed frequency. Sometimes we can add other medications to the biologic to see if this can improve symptoms. In particular we may try methotrexate if this had been ceased or sometimes we can try adding in some of the other non-biologic drugs. It is really frustrating when someone has been fantastic for 12 years and then suddenly the drug stops working.
Naomi: What is the record for some of these biologics working really well for?
Rachelle: We’ve had Enbrel® and infliximab since 2003 in Australia and there’s certainly some patients who have been on the same one since that time. I know a gentleman who has been on infliximab right from the word go and others who have been on Enbrel® or Humira® since that time as well.
Naomi: And is there much evidence of effective use of these medications specifically in ankylosing spondylitis?
Rachelle: We have specific drugs available for ankylosing spondylitis called anti-TNF drugs and yes there is strong evidence that they are very effective for ankylosing spondylitis in terms of relieving the pain and helping with function and also some recent evidence that they can slow the progression of the disease and stop the ankylosis (stiffening or fusion of the spine). Often these drugs make a significant difference to people’s lives.
Naomi: Do biologics affect hormone levels in any way? In particular testosterone and cortisol?
Michael: I am not aware of any evidence around testosterone in particular. Cortisol is a little bit more complex in rheumatoid arthritis patients because they might be taking other medicines like prednisolone that might affect cortisol levels. But again I am not aware of any evidence that biologics might have an adverse effect on cortisol levels directly.
Naomi: What about kidney health and liver health? Do the biologics affect these organs?
Rachelle: Not generally, no.
Naomi: So the risks with biologics are very much similar to other medications that people take to control their disease. We have had a few questions from people asking about risks. About whether there are any more dangerous than other drugs. What can you say about that Michael? Compared to other medications, are they more risky?
Michael: The statement around are they more risky than other medications, it’s a little bit more tricky in that all therapeutic decisions are about what are the potential benefits of a particular medicine versus the risks and making sure there is sufficient benefit to justify their use. You know these drugs they do suppress the immune system and when you do suppress the immune system that brings with it some predictable types of risks. There are some potential types of risks around infections and some other things and so acknowledging those risks is important and then it is about being proactive and making sure we use the right measures to try to minimise that risk. So I’d be less inclined to put it are these riskier, its more about understanding what the risks are and then trying to reduce those risks as much as possible.
Naomi: I guess as patients we do have to weigh up those risks and rheumatoid arthritis and other inflammatory arthritis’s can be very damaging on people’s lives and sometimes it’s worth giving these medications a go and seeing if they can have a positive impact on your life.
Rachelle: As I said we run a national database. We have been following patients since 2003 and periodically we perform various analyses of the data to look at the long-term benefits of the biologics and also look to see if there are any serious risks. We have done a couple of linkages to the Australian cancer registries and it is reassuring that we have not found any overall increased risk of malignancy. We were worried about increased risk of skin cancer but we have not been able to demonstrate any increased risk of those related to biologics either. We do know that they might increase the risk of infection or delay getting over an infection so if people get really sick with an infection we would generally stop the biologic medication (and some of the other medications like methotrexate) until they are better. However pleasingly we have not really found any other major signal that these drugs cause problems over the longer term.
Naomi: What about people who are considering having a family? What do they need to know about biologics? Can they be on these before getting pregnant, and after and during breastfeeding? And also males, what do they need to consider as well?
Rachelle: For males we don’t think it’s a problem that they can be on the biologics. In females, there is an increasing body of evidence looking at their safety during pregnancy and breastfeeding but it takes time to build the evidence base as it requires lot of women having experience of being on these drugs during pregnancy. And so, the guidelines have gradually become more relaxed and some of the drugs are considered safe to take at least through the first trimester. They also seem to be safe during breastfeeding. I think that is an evolving field.
Naomi: What other medications were there that people have been on during pregnancy? You mentioned Enbrel®, what are some of the others?
Rachelle: Most of the evidence for pregnancy actually comes from people who have other conditions like inflammatory bowel disease where the drugs have been used throughout the pregnancy. These are the TNF inhibitors – Enbrel®, Humira® and infliximab. We don’t yet know enough about the safety of the newer anti-TNF drugs or the other types of biologics during pregnancy.
Naomi: We have a live question: Can biologics such as Humira® have an adverse effect on other medications such as anti-depressant medication?
Michael: I mean this is an interesting topic. In general we would say that biologics don’t interact with other medicines. That because they’re very different in their nature compared to traditional medicines – Rachelle showed us before the very small aspirin molecule and the very big biologic molecule. The ways that the drugs normally interact with each other don’t apply in the case of the biologics. But there are some potential effects that could result in drug interactions with biologics, between some medicines and the biologics. So I think with any medicines it’s best to tell all of your health care practitioners, your doctor, your GP, your pharmacist, anyone you’re seeing about starting a new medicine and just telling them you are taking a biologic. So they can make sure there isn’t a potential interaction.
Naomi: Rachelle do you have something to add to that?
Rachelle: No I agree. I am not aware of any major interactions.
Naomi: Are children given biologics or biosimilars?
Rachelle: Children with juvenile idiopathic arthritis are also able to be treated with biologics. We also follow them in our national registry, as well as rheumatoid arthritis and ankylosing spondylitis.
Naomi: What is the success rate of Humira® for rheumatoid arthritis as opposed to using Enbrel® and Xeljanc®?
Rachelle: From our ARAD studies and other databases and our systematic reviews where we have synthesised all the trials together, Humira® seems to work just as well as Enbrel® and their safety profile is also very similar.
Michael: One point I would like to add is some people respond better to one than the other. So if we look at everybody they work about the same. But that doesn’t mean that for any given person both medicines would work the same. Some people might work a little bit better with one or the other. Unfortunately we can’t quite predict who that is at the moment. So sometimes it is a little bit of a trial and error to see which one works best for each person.
Rachelle: Certainly those are the most popular biologic drugs prescribed in Australia. Humira® is slightly more popular, but I think that is because the injections are every two weeks and the Enbrel® injections are every week.
Naomi: Do biologics have any effect on insulin action and blood glucose levels?
Michael: Again with some of these things we need to be a little bit careful because sometimes patients with rheumatoid arthritis will be taking other medicines at the same time that can make the blood sugar levels change and so it may not necessarily be the biologic. It might be something else they are taking sometimes.
Naomi: I think we will go to talking a little bit more about biosimilars now. Rachelle has described what they are and what the difference is between biologics and biosimilars. People have been asking about their accessibility.
What is the accessibility like for, or the eligibility for biologics and what does a patient need to do to be able to get onto a biologic or a biosimilar?
Rachelle: Biosimilars in Australia are considered completely interchangeable with the biologic that they are similar to. At the moment there are two biosimilars for infliximab called Inflectra® and more recently Renflexis®, and there is one biosimilar for etanercept called Brenzys®.
The criteria that a patient has to fulfil to be able to have these drugs prescribed by their rheumatologist is exactly the same as for the biologic. The Australian Rheumatology Association (ARA) concurs with the Australian Government that if people have not had any biologic before then it is very reasonable to start them on a biosimilar because they are cheaper and this means that we will be able to afford more medicines on the Pharmaceutical Benefits Scheme (PBS). We don’t see any downside to doing that. If someone is already on a biologic, so if they are already on Remicade® or Enbrel®, then we’re a little bit less comfortable switching to a biosimilar because we still do not have enough information about multiple switches and whether or not this might increase the chances of secondary failure (the drug works well initially but then stops working later). Switching between similar but not identical medications might induce an immune process. So we don’t really know. So at the moment if you’re already on a biologic and its working well for you, then we (the ARA) suggest you stay on it. Similarly if you are started on a biosimilar then we also think it might be better to continue on it.
Michael: You have introduced some very important topics there around switching. I think we perhaps might benefit from going back and having another look at those slides again. Just briefly we will talk a little bit more about what a biosimilar is and how it is evaluated.
So on this slide again that Rachelle showed us, on that right hand side we have that very large molecule representing the type of biologic medicine that a patient with rheumatoid arthritis and other inflammatory arthritis’s might be taking. And you can see that it is a very large structure. It is very complex and then if we look on the left hand side in that little box in that bottom left hand corner, that little structure, that little hexagon with some antennae hanging of it that’s actually aspirin. You can see that’s very small, it’s very simple, so it’s very easy for two different companies to produce exactly the same molecule, that same structure.
And we have techniques in the laboratory which allow us to determine if two different companies are making exactly the same thing. A little bit like looking at a finger print. We can check that the finger prints are identical. And if the finger prints match we call that a generic medicine.
But look at the structure on the right. If we look at the biologic on the right hand side we can see that it is a large structure, it is very complex. You can see that little aspirin molecule disappears into the enormous structure of that biologic and our techniques in the laboratory to investigate those structures and determine those fingerprints, they’re just not as good. We can’t quite get a good picture of it because it is just so complex. And so, because we can’t prove with absolute certainty that two different manufacturers of a biologic medicine are making exactly the same structure, that’s why we have this term biosimilar.
We are not saying they’re identical. We are saying there might be some slight differences and so they have to go through a process of evaluations. That process starts out by the company that wants a biosimilar version of the original product. They start out by trying to look at that structure as best they can in very high, fine detail and they spend a lot of time and money going through those structures [asking] “how well does my copy version compare to that original molecule?” And if they’re confident their molecule is a good copy, a good replication, they then proceed to the next stage which is to look at how it affects cells. [They ask] “Does it produce the same biological effects?” And if it does, then they say: “… now it not only looks like it has a very similar structure, but it also has the same biological effects.” Then they say: “Let’s then take that and put it into people. We will start out with healthy people and we will look at what we call pharmacokinetics. So, we inject the drug and we see what happens. We see if the concentration goes up and down over time and we compare does the potential biosimilar produce the same pharmacokinetics that we would see in the original product.” If they look the same, then the final step in getting a biosimilar to the market, or to get it approved for use in Australia, is to run a clinical trial. To take the potential biosimilar and the original brand of the product and do a clinical trial in patients with the disease. It might be RA, it might be another condition, and say: “if we give each of those two products to different groups of patients what do we see? Does the biosimilar produce the same safety and efficacy that we would see from the original product?”
That’s a slightly smaller trial than what was originally done with the brand of the product. But we’ve got a lot of information about how this drug works, so what we’re trying to see is: how does this new copy version compare to the other [original] version? From that you then develop evidence and say well this drug looks like its structure is the same, it behaves the same in cells, it behaves the same in healthy people and then when we finally get patients, it behaves the same as well. But what we haven’t done is turned around and said: “well if we start a patient on the original brand and change them over to the biosimilar what happens?” And that is what is called switching, that is what Rachelle referred to.
Naomi: What are the risks then for someone switching if they have been stabilised on a medication, on a biosimilar, and then are switched?
Michael: That’s a great question. So the concern has been that because these are very large molecules your immune system can recognise them and Rachelle spoke about how the immune system can recognise the biological medicine and when it does that you can lose response to it. And that’s not a good outcome. That’s not what patients want so the concern has been that because there might be very subtle differences between the biosimilar and the original brand of the product then maybe that would influence the risk of those sorts of reactions. And so, the concern has been that well if we don’t have evidence about switching it may not be safe.
[However]… we haven’t seen any increased risk of immunogenetic reactions. We haven’t seen a whole lot of people develop what we call anti-drug antibodies. That is the immune system recognising the biologic medicine after they have changed from the original brand to the biosimilar. There was a study in Norway called the NOR-SWITCH study which looked at this in that country and they didn’t find anything of any concern. There has also been some other registry studies. Rachelle referred to the ARAD registry, a very important registry. And there are other registries around the world where they have looked at the impact of switching biosimilars… And again they haven’t raised any concerns. But it’s still relatively early days. We are seeing one way switches where patients go from the brand to the biosimilar, but we haven’t necessarily seen lots of switching back and forth. So a patient may go from brand to biosimilar to brand back to the biosimilar.
Naomi: And that would likely happen at a pharmacy level, where they switch back and forth?
Michael: Potentially, yeah. So that raises another question, so …
Naomi: I was going to talk about substitution and switching. They are slightly different.
Michael: They are slightly different things. So in one mechanism it could be that the prescriber says, “Ok, let’s change from the original brand to the biosimilar”. This is done with the patient and the doctor being aware. And that’s one way of doing it. And the other way in which it could happen in Australia is what’s called substitution – where the doctor writes the prescription, the patient takes it to the pharmacy, and the pharmacist gives them the alternative brand, the biosimilar version. That would be referred to as substitution.
Naomi: So what do you think patients need to do to make sure that this doesn’t happen at the pharmacy level? Well not that it doesn’t happen as it might be something that’s intended to happen, but what should they do to make sure they keep abreast of their condition and their medication. What do you recommend?
Michael: The way I’d like to describe all of this is shared decision making. It’s about everyone being informed. There are potentially significant benefits of biosimilars. These medicines are very expensive and the need to bring down the cost of medicines is really important. If we can bring down the cost across any area of treatment it’s better for everyone else. We can provide treatments earlier to other patients who are in need of life changing therapy. But again it has to be in the context of individual patients and that’s why I say it’s about shared decision making: a discussion between the patient and the prescriber about whether a biosimilar may be right for them.
At this point there is no evidence to suggest that changing from the original to the biosimilar causes harm but some patients may not feel comfortable with that because it is complex and there is a degree of uncertainty and that may cause them to be stressed and that’s not a good thing. So again it should be a discussion between the patient and their prescriber. And then when it goes to the pharmacy it might be a matter of, or it should be, a discussion between the patient and the pharmacist about what they’re going to dispense for them. And there are a whole range of considerations. For example as we have said these are very expensive medicines and pharmacies can’t afford to keep all brands of all medicines. It’s just not possible because of the expense. So it might be that the pharmacy chooses to stock one particular biosimilar and if that’s all they have access to at that point in time – again, it’s about what the patient can access. So again it’s about the decision. And it’s completely appropriate for the patient to say “No, I don’t want the biosimilar”. And similarly, at the discussion with the prescriber. There are some things that can be done if the decision is to only use the original brand. Then there are ways to make sure that happens.
Rachelle: Firstly, if the box on the script titled “No brand substitution” is ticked then the pharmacist is not able to switch. They must dispense the same drug. And many of my patients, I know they put the script in and then the pharmacist orders it in. What we are really concerned about is if the rheumatologist doesn’t tick that box and the patient does get a different drug that we don’t know about and if the patient’s [new] drug was causing an affect and we don’t know what drug the patient was taking. This is why we have our national database to keep track. So it’s really important that if switching does occur that the patient is fully informed and knows that and that they go back to their rheumatologist and take their [medication] packet with them and show them that the drug has been switched. So that we can monitor them knowing that they have switched.
If patients have never been on a biologic, it would be very reasonable as Michael says to use the biosimilar. In this way we’re helping society by lowering the cost of the drugs more generally. But it’s also important to note what I said at the beginning, that even one batch of a biologic can be slightly different to the previous batch. So the difference between the biologic and the biosimilar might be no different than the difference between one batch of a biologic and another batch of the biologic. And maybe that is some of the reasons why the drugs stop working as well. But again we don’t know. But the most important thing is that patients understand that their medication has been switched and that they are fully informed of the possibilities and that their rheumatologist knows.
Michael: I think there’s one other practical consideration that comes into this idea of switching and substitution. These are complex medicines that we can’t give as a traditional tablet because [in such a format] they won’t work. They have to be given as an injection and for many of these drugs it’s given as an injection that patients use at home. Injections use a device and the device will be different between the biosimilar and the original brand and that’s important for patients to be aware of. The last thing I think that any pharmacist would want is for a patient to get home and suddenly discover “oh this product looks different, I don’t know how to use this.” So, again as part of that decision making … a part of that [discussion] should be that the device will look different and that the device functions differently. That’s not to say that the biosimilar is inferior in terms of the device. In fact some of the literature would suggest that because [biosimilars] are made or the devices they are being used to inject with are … newer – … in some instances [they] could conceivably be easier to use. That’s a slightly different discussion but the point is that patients need to be aware that the device of a biosimilar might look different and [they] need to understand how to use that device. That’s really important. And [that biological medicines are] really different from traditional medicines which are just a tablet. A generic medicine, it doesn’t matter what shape or colour the tablet is, you still swallow it the same way. But an injecting device, that functions in slightly different way.
Naomi: Would patients learn about injecting devices that may differ between the biologic and the biosimilar from their rheumatologist or would they talk to their pharmacist?
Michael: So again, it depends on the setting. If you know it was a switch that was initiated at the level of the prescriber than it might occur there, but if its substitution by the pharmacist without the knowledge of the prescriber then it is absolutely incumbent on that pharmacist to provide the appropriate training around the use of that device. That is their responsibility and it can be done. These devices are reasonably easy to use. But it’s about ensuring the patient is properly educated. And if the patient isn’t comfortable with that new device then maybe that’s not the right thing to be doing at that time.
Naomi: Some biologics are a given in hospital settings through intravenous therapy (IV), what should patients be aware of because they’re not actually usually given something over the counter. What should they be looking for or ask questions about – with just keeping on top of what’s being put into them?
Michael: Rachelle might know.
Rachelle: Different hospitals have different stockists and so some hospitals may only keep one brand of a biologic or biosimilar. If you are on an IV biologic then you need to ask your doctor, whether or not your infusion will be the same one as the one you have been taking. Similarly you can ask your doctor to inform you if the hospital changes to a biosimilar from a biologic (or vice versa).
Naomi: So is there actually a risk of patients not knowing a switch occurs in a hospital setting?
Rachelle: Absolutely, yes.
Naomi: So it’s just asking the right questions of the doctors … and even having a look at the bag to see what name it has on it.
Michael: I think it’s about asking the right questions. I mean, in the hospitals doctors are all very aware of the biosimilar discussion and so…
Rachelle: And in those instances we would probably be a little bit less concerned because it would only be a single switch. So unless they change hospitals or change rheumatologists in hospitals, then they would continue to get whatever they have been switched to and its unlikely they will go switching backwards and forwards unless the hospital has an annual policy of looking for the cheaper brand. But I don’t know if that would happen. Michael might know that.
Naomi: I’ve got another question here – if more patients take biosimilars in the future does this mean that rheumatoid arthritis sufferers will be able to get on these drugs more quickly without waiting to try other drugs first? For example, when they present with severe rheumatoid arthritis will they be able to go straight onto a drug that will work well for them. This person had to wait about 9 months to get onto Enbrel® and have irreversible joint damage because of this. So, we patients need to get onto these as quickly as possible.
Rachelle: Many people will also do very well on the older traditional disease-modifying drugs. Not everyone with rheumatoid arthritis need to go on biologics. When drugs are listed on the PBS, the committee that decides (the PBAC) looks very carefully at the evidence and makes decisions based upon the effectiveness, safety and cost-effectiveness of the new medicines in comparison to what is already available. So biologics as first line treatment might be the right choice for some people but not others. The government hopes that if more people are prescribed biosimilars then this will drive down the prices of both biologics and biosimilars.
Michael: I think that’s a very important point. We have a very rigorous process for evaluating biosimilars in this country. It’s not just a matter of making a copy and sending it to the market. It is a very rigorous process and people are looking at the evidence around the biosimilars intensely to make sure there is no potential signs of harm. And as long as we continue to do that the potential benefits of reducing treatment costs are enormous. It might be there are ways of providing access to particular patient because not all patients need to go to a biologic. But [for those patients that do] … we could provide easier access or we can afford to spend on other medicines and there are some good examples in the literature. There is an example from overseas where a hospital switched to a biosimilar infliximab and they were able to use some of their savings from that change to help pay for additional nursing support. That made an enormous difference to their patients. However, I do think we need to look carefully at what the literature around biosimilars says and all the literature we’re seeing is reassuring and then look at ways we can maximise the potential savings for better health care more generally.
Naomi: So the cost savings may not necessarily directly relate back to patients with these diseases but in helping the health system more generally. Helping the government put it into other areas of improving health for people.
Michael: I mean it might be these patients or it might be other patients. At the end of the day the government pays an enormous amount for these patients.
Naomi: We’ve got time for one or two more questions. If a patient has been on several biologics over 6+ years but blood inflammation levels (ESR and CRP) remain high e.g.: 35-40 for over a year, what is the probability of the government withdrawing the subsidy if these levels are still high and the drugs are not working for them?
Rachelle: If it’s not working for them then there are other drugs that might be better able to control their disease. I guess that is a question for their rheumatologists. The government isn’t trying to take drugs away from patients, they’re trying to do the best they can within the limits of a fixed health budget. So this really depends upon us using these new drugs responsibly to ensure that those patients who need them can have access to them.
Naomi: Unfortunately we are out of time we are going back to Ornella.
Ornella Clavisi (Musculoskeletal Australia): Thank you for listening in today. I would like to thank our panel and our audience for participating and submitting questions, and I would also like to thank Abbvie for sponsoring this webcast. Thank you, thanks everyone.
Diagram 1
This webcast was made possible through an unrestricted educational grant from Abbvie.
![]()








