MeTeOR2 Clinical Trial
July 1, 2024 by Musculoskeletal Health Australia

MeTeOR2 Clinical Trial
The MeTeOR2 Clinical Trial compares meniscal allograft transplant surgery to a type of physiotherapy for people who experience post-meniscectomy pain.
Clinical trials
Clinical trials are an important part in improving healthcare outcomes. If you are not familiar with what a clinical trial is, you’re not alone! Clinical trials are research studies involving people like you and me, to evaluate if a treatment is safe and helpful. The new treatment under testing may be a medicine, procedure, technique, vaccine, device, or lifestyle/behaviour. Clinical trials are carefully designed and must undergo a thorough review to ensure the procedures have merit, are safe and ethical. Ultimately, clinical trials are essential to help us improve the management of health conditions!
People participate in clinical trials for various reasons. Some people who are aware of clinical trials understand their participation helps inform how to manage health conditions. However, many may not be aware of the other benefits. These benefits include playing an active role in your healthcare, receiving treatment from leading medical practitioners, and accessing treatments for your condition which may not be possible otherwise. One clinical trial is the MeTeOR2 trial, which compares meniscal allograft transplant surgery to a type of physiotherapy for people who experience post-meniscectomy pain.
What is a meniscectomy?
The meniscus is a c-shaped cartilage that distributes loads at the knee joint. A torn meniscus is common, especially among young people engaged in sport activities. Conservative treatments are available including activity modification, medication (e.g., non-steroidal anti-inflammatories, paracetamol), and physiotherapy. Some tears may be surgically repaired depending on the location of the tear. Other tears cannot be repaired, so the torn parts are removed with a keyhole surgery called arthroscopic partial meniscectomy. Although this procedure can improve pain and locking symptoms in most people, some patients may still experience pain and functional limitations after the surgery. This scenario can be very disabling and emotionally distressing, particularly at a young age when people are expected to be active, healthy, and free of disability. When this is the case, only a few treatment options are available.
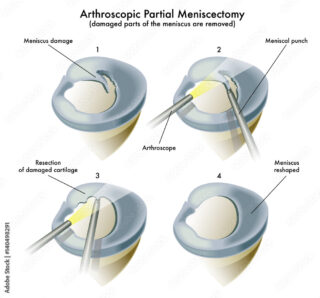
Treating pain and functional limitation after a meniscectomy
For people with considerable pain or functional limitations after meniscectomy, surgical and non-surgical treatment options are available. One surgical option is the meniscal allograft transplant (MAT), which involves inserting a donated meniscus into the injured knee. The MAT surgery is thought to improve symptoms by restoring normal load distribution within the knee, along with the rehabilitation after surgery. Non-surgical management, such as personalised rehabilitation – which consists of many different types of treatment, including exercise, weight management, and lifestyle and activity advice – is also a viable option. Although the mechanisms of how personalised rehabilitation reduces symptoms are unknown, improvements have been observed in people with post-meniscectomy pain. However, it is still unknown which treatment option is better, in terms of safety and cost-effectiveness, to improve health-related outcomes.
The MeTeOR2 study
MeTeOR2 is a clinical trial designed for people suffering from pain and/or functional limitations after partial meniscectomy. This study aims to compare two knee treatments – meniscal allograft transplant (MAT) surgery and personalised knee therapy (rehabilitation) – and evaluate if one treatment is superior to another in improving knee pain and function.
How to participate/who to contact
If you are interested in the study and consent to participate, you will have a 50% chance of being put into one of the two groups. The first group will receive the meniscal allograft transplant (MAT) surgery, while the second group will receive personalised knee therapy (rehabilitation). Participants in both groups will be asked to complete a series of questionnaires at baseline and 3, 6, 12, 18, and 24 months following randomisation.
You may be eligible to participate in this study if you have:
Inclusion Criteria:
- Pain and / or functional limitation from the knee, severe enough to warrant potential meniscal allograft transplant.
- Previous meniscectomy ≥ six months ago.
Exclusion Criteria:
- Symptomatic ligament instability, not previously corrected, as determined by the assessing clinician.
- Coronal limb alignment that requires surgical correction, as determined by the assessing clinician.
- Age < 16, or if ≥ 16, open growth plate at the proximal tibia as judged by the clinical team on imaging as part of standard care.
- Full thickness cartilage loss (exposed bone) > 1 cm2, on routine clinical MRI, prior to surgery, or any other form of clinical imaging or evaluation.
- Inflammatory arthritis affecting the study knee as determined by the assessing clinician.
- Unable or unwilling to engage in rehabilitation.
- Unable to adhere to trial processes.
- Previous randomisation in the present trial, (i.e., the other knee).
If you are interested in being part of this important and exciting clinical trial, please contact the study facilitator: meteor2.study@sydney.edu.au
Contact our free national Helpline
Call our nurses if you have questions about managing your pain, musculoskeletal condition, treatment options, mental health issues, telehealth, or accessing services. They’re available Monday to Thursday between 9am-5pm on 1800 263 265; email (helpline@muscha.org) or via Messenger.








